CYTOKINETICS’ new heart treatment, Aficamten shows amazing results in testing. This could change the way we treat heart problems completely. The focus on how cells move is talked about in important studies, suggesting a possible change to the way we treat heart problems.
Aficamten by CYTOKINETICS Shines in SEQUOIA-HCM Trial
Improving hearts, Beat by beat
In the area of block heart muscle growth (HCM), Aficamten by Cytokinetics is a big change. The big study called SEQUOIA-HCM shows that exercise ability and relief from symptoms greatly improved for grown-up people. This special heart muscle blocker not only makes breathing in more air better but also beats other things with no bad effects.
Unveiling the Impact on Heart Function
Aficamten is good at useful heart tests. It makes the chances of your main pump changing less than 50% lower. This is very different from the normal treatments that don’t directly deal with heart problems. Dr. Malik points out that heart muscle blockers like Aficamten directly deal with the main cause of HCM, raising a new level in its treatment.
Anticipated Revelations and Future Prospects
While we have to wait until 2024 for the whole SEQUOIA-HCM trial results, another study called MAPLE-HCM is looking at Aficamten as a treatment right from the start. Another test, ACACIA-HCM looks at how well Aficamten works for non-blocking HCM. It gives a full picture of possible uses of this treatment.
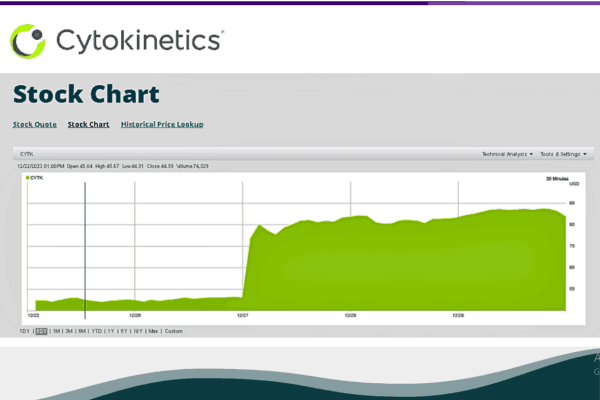
Wall Street Rallies as CYTOKINETICS Shares Soar
Market’s Vote of Confidence
The effect of Aficamten‘s success is felt on Wall Street, as CYTOKINETICS shares jump by 82%. People who study the stock market think things will improve. They are excited about a company called CYTOKINETICS and believe in its success because of good results from something named SEQUOIA. This has boosted Barclays, UBS, and JPMorgan‘s enthusiasm for this business. The increase means more money, but it also shows the possible usefulness of Aficamten.
Analyst Insights Paint a Promising Picture
Barclays increases its price goal, focusing on Aficamten‘s strong chances. Meanwhile, UBS keeps a Buy rating saying the results are just short of the best possible situation. JPMorgan thinks CYTOKINETICS‘ shares might be good until 2024 because they could have real value.
Aficamten of CYTOKINETICS Triumphs in Heart Disease Study
A “Home-Run” for Heart Health
CYTOKINETICS does very well with Aficamten, winning in an important late-stage test. People are praising it as a big rival to Bristol Myers Squibb‘s Camzyos. They like its performance and how well it works better than others. The big increase in the stock shows that investors believe, maybe giving billions more value to CYTOKINETICS.
CEO’s Optimism and Market Impact
CEO Robert Blum is happy with the statistics, exceeding what was hoped. This leads them to try for approval from U.S marketing teams. People with heart muscles that are stiff will get relief due to its ability to aid the heart muscles during exercise. This shows it could be one of the best kinds around, making big drug companies interested to buy it out.
The Road Ahead: Journey of CYTOKINETICS to Redefine Heart Care
With good test results, high shares in the market, and positive signs from experts, CYTOKINETICS‘ drug Aficamten has a bright future. As the company deals with rules and expects more tests, attention on CYTOKINETICS gets bigger. This suggests big changes in how heart problems are treated. The path from experiments to marketing might show a change, with Aficamten being seen as hope for those dealing with heart problems.
This could change the way we treat heart problems completely. The focus on how cells move is talked about in important studies, suggesting a possible change to the way we treat heart problems.