In recent years, weight loss drugs, specifically GLP-1 agonists such as Semaglutide, Tirzepatide, and Liraglutide, have gained popularity for their effectiveness in helping individuals lose weight. However, a comprehensive study conducted by researchers from the University of British Columbia has uncovered concerning gastrointestinal side effects associated with these drugs, particularly when used by individuals without diabetes.
U.S. FDA Warnings On Weight Loss Drugs
Concerning the widespread use of Weight Loss Drugs, the U.S. Food and Drug Administration (FDA) has already issued warnings about the potential risk of intestinal blockage with semaglutide drugs, following thousands of reports of the condition from both diabetic and non-diabetic users. This new study delves further into the risks posed by GLP-1 agonists when used solely for weight loss.
The Comprehensive Study Report on Weight Loss Drugs
The study, published in JAMA, analyzed data from approximately 16 million U.S. patients, focusing on non-diabetic individuals prescribed GLP-1 drugs and a comparison group on different weight loss medications. The results revealed that those on GLP-1 Agonists faced significantly higher risks of Pancreatitis (nine times higher), Obstructed Bowels (four times higher), and Gastroparesis (a condition involving delayed stomach emptying into the intestines).
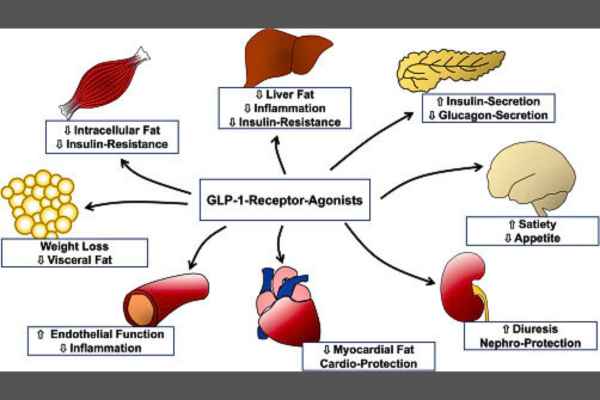
While GLP-1 Agonists have demonstrated benefits in helping individuals with diabetes manage their blood sugar levels, the gastrointestinal risks may outweigh the benefits for those without diabetes. Although some studies have suggested positive outcomes, such as a reduced risk of heart disease, further research is necessary to confirm these findings.
These Weight Loss drugs, which include Wegovy and Ozempic, have been widely praised for their ability to facilitate substantial weight loss over time. They function by slowing down digestion, curbing appetite, and prolonging feelings of fullness. However, this slowing effect can lead to adverse gastrointestinal effects, including abdominal pain, nausea, vomiting, and, in severe cases, Stomach Paralysis (Gastroparesis).
The study primarily focused on Semaglutide and Liraglutide, comparing them to another weight loss treatment called Bupropion-Naltrexone, which operates differently to assist with weight loss. While Wegovy is explicitly approved for weight loss in the U.S., Ozempic, sharing the same active ingredient as Semaglutide, has been approved since 2017 and is occasionally prescribed off-label for weight loss.
The Manufacturers Take On Weight Loss Drugs
A spokesperson for Novo Nordisk, the manufacturer of Wegovy, Ozempic, and similar drugs, reiterated that some of the gastrointestinal side effects mentioned in the study were already listed on the drugs’ labels. They emphasized the safety and efficacy of these medications when used according to product labeling and approved indications. Patients were advised to use these drugs under the supervision of healthcare professionals who could evaluate their medical profiles and weigh the potential risks against the benefits.
Conclusion
This comprehensive study sheds light on the serious gastrointestinal risks associated with GLP-1 agonists when used exclusively for weight loss. As concerns grow regarding the side effects of these drugs, especially for non-diabetic individuals, thorough evaluation and consideration of the risks and benefits are crucial. Patients are encouraged to consult healthcare professionals for personalized guidance on the use of these medications.




